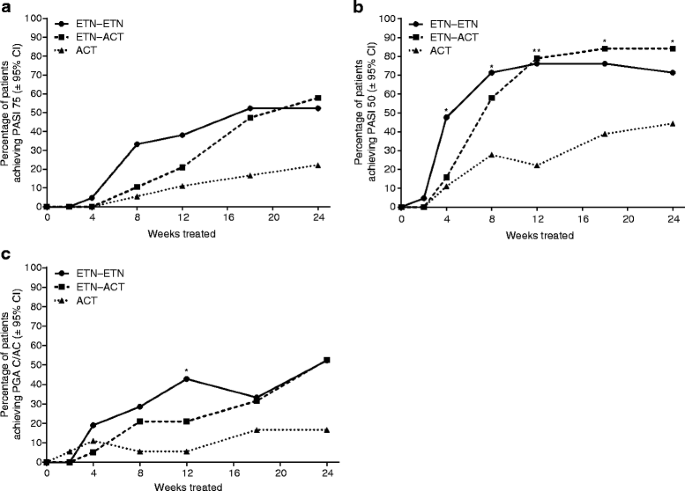
A multicenter, randomized, open-label pilot trial assessing the efficacy and safety of etanercept 50 mg twice weekly followed by etanercept 25 mg twice weekly, the combination of etanercept 25 mg twice weekly

JCM | Free Full-Text | Use of Guselkumab for the Treatment of Moderate-to-Severe Plaque Psoriasis: A 1 Year Real-Life Study

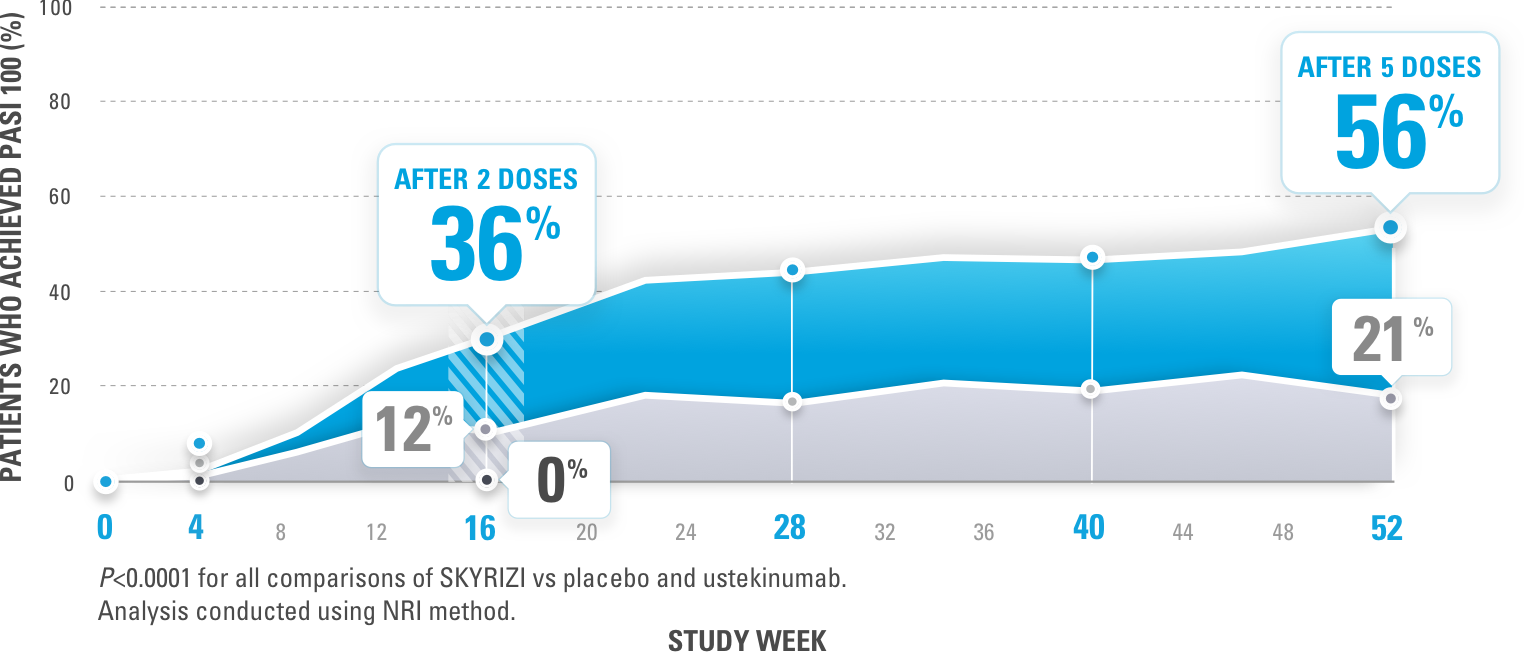
Figure 2 | Exposure–Response Relationships for the Efficacy and Safety of Risankizumab in Japanese Subjects with Psoriasis | SpringerLink

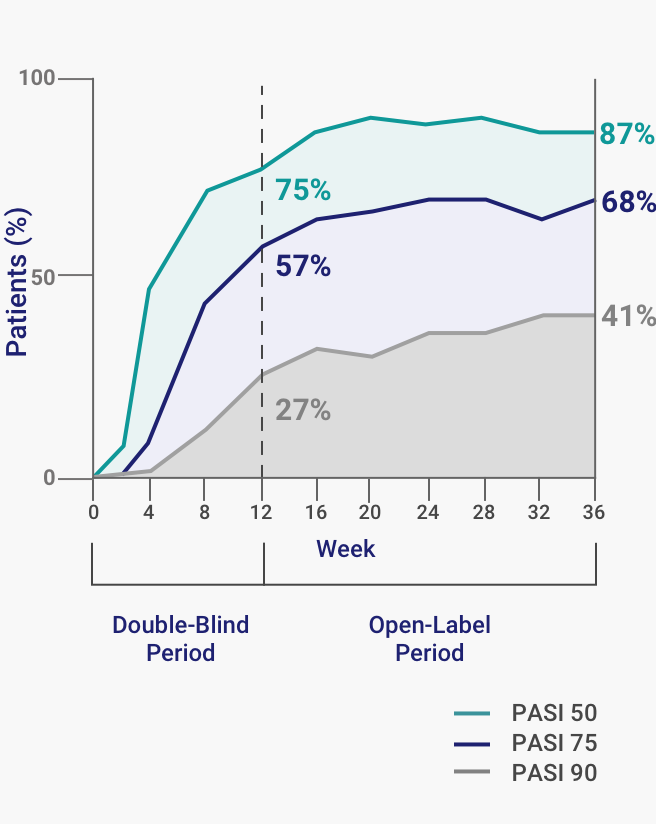
A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis - ScienceDirect
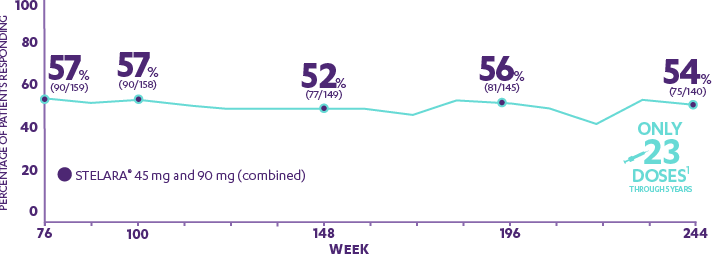
Novartis' Cosentyx™ two-year data shows sustained effect and favorable safety profile in psoriasis patients | Novartis
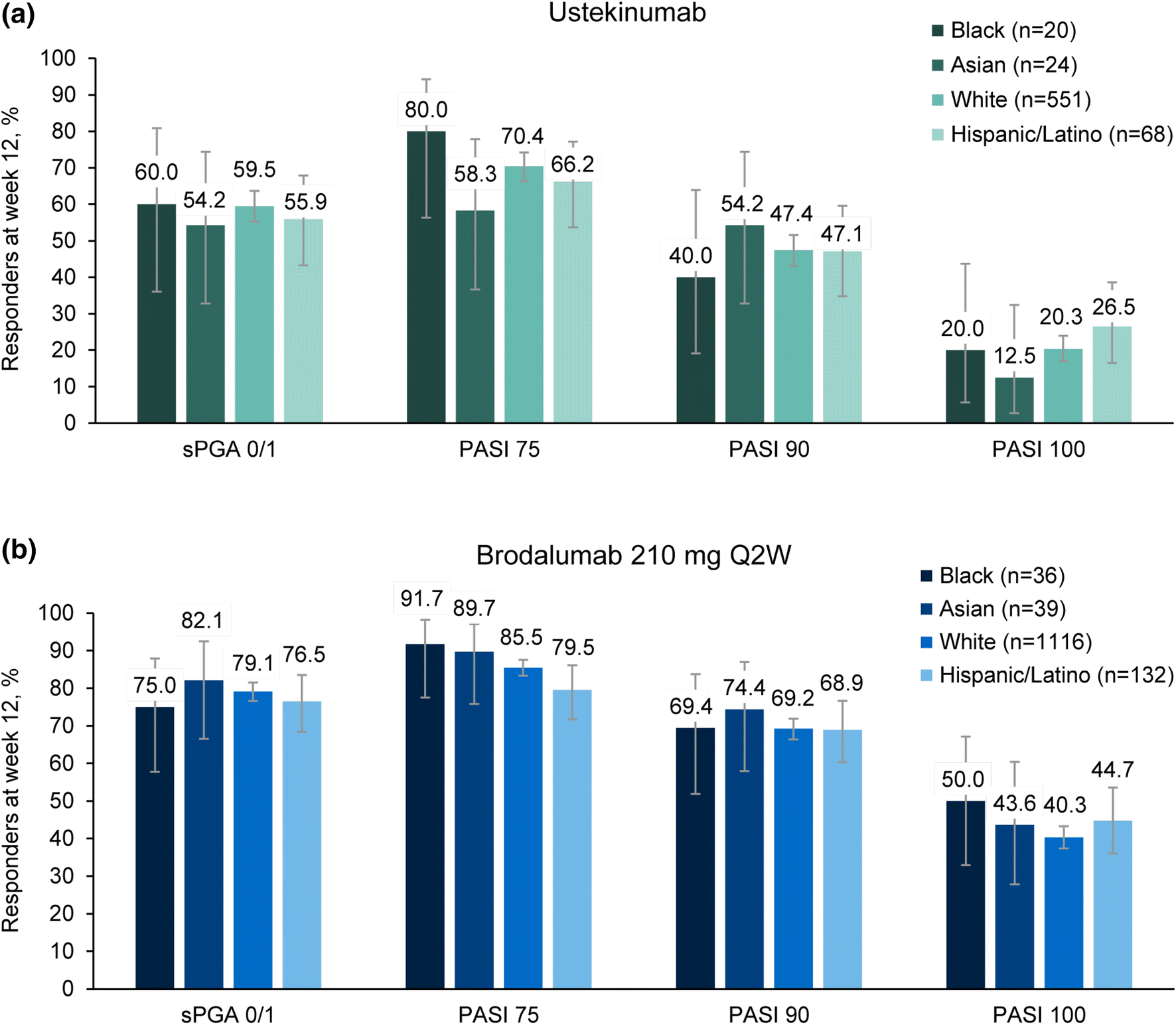
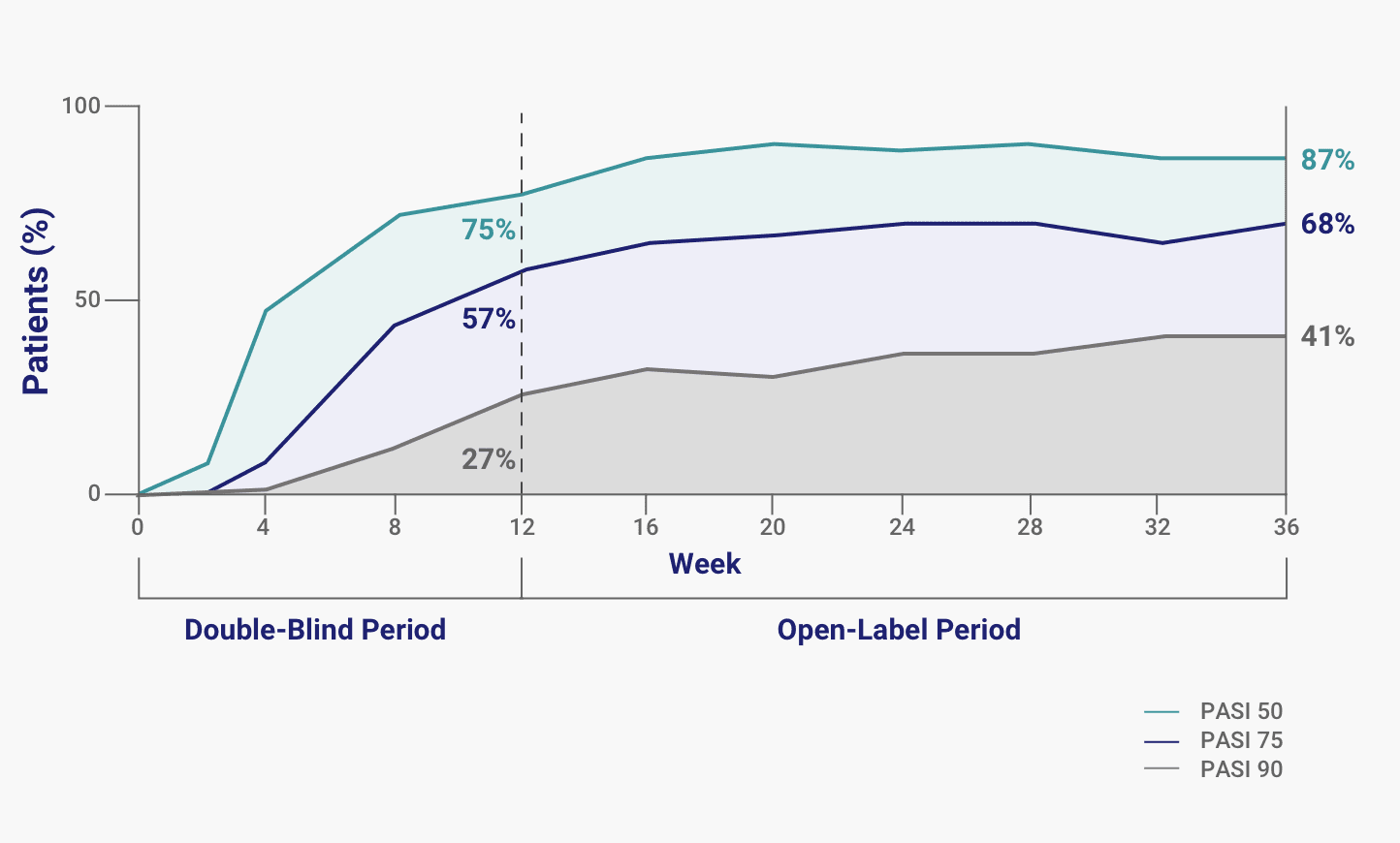
Figure 2 | Efficacy and Safety of Brodalumab in Patients with Moderate-to-Severe Plaque Psoriasis and Skin of Color: Results from the Pooled AMAGINE-2/-3 Randomized Trials | SpringerLink

Improvement in Psoriasis Symptoms and Physical Functioning with Secukinumab Compared with Placebo and Etanercept in Subjects with Moderate-to-Severe Plaque Psoriasis and Psoriatic Arthritis: Results of a Subanalysis from the Phase 3 Fixture

Proportions of patients who achieved (a) PASI-75 and (b) PASI-50 and... | Download Scientific Diagram

Time course of PASI 75, PASI 90, sPGA 0/1, and DLQI overall score of... | Download Scientific Diagram

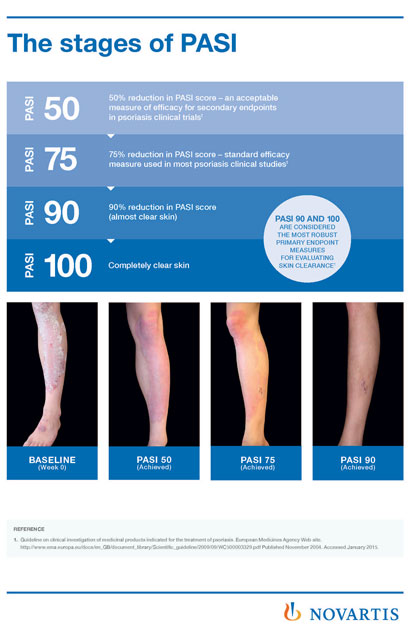
Perspectives in Psoriasis: Assessing Treatment Efficacy—Which Measures, What Do They Tell Us? - ppt download
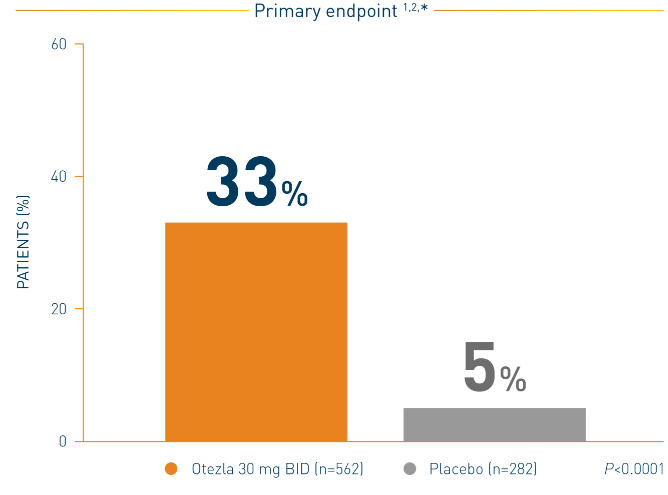
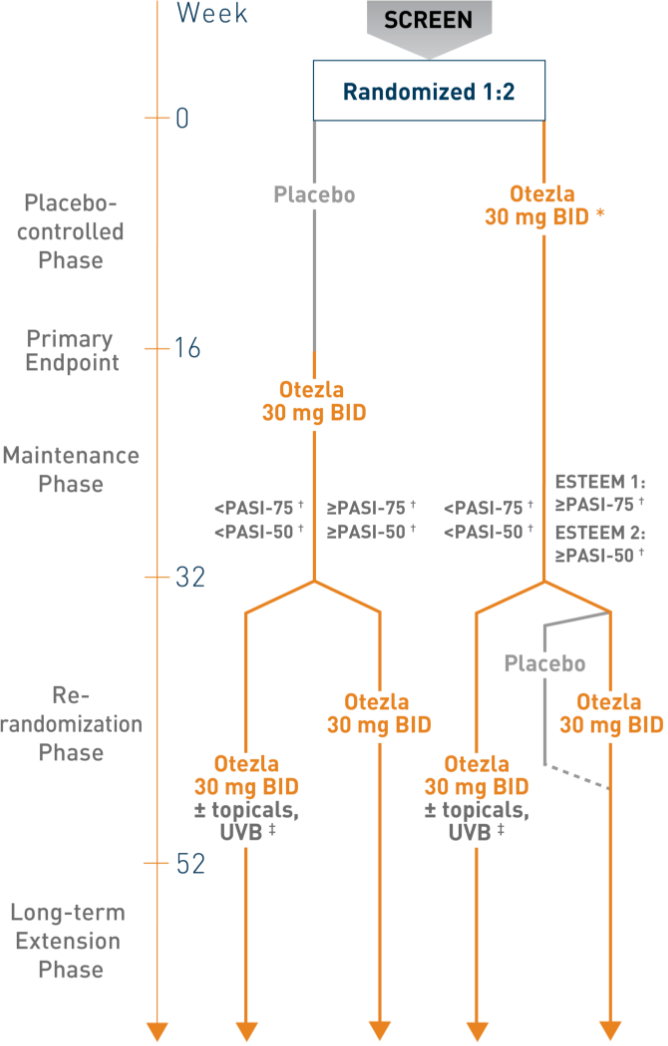
PASI-75 Response with Otezla in the Treatment of Moderate to Severe Plaque Psoriasis — Efficacy | Otezla® (apremilast) Healthcare Professional Site
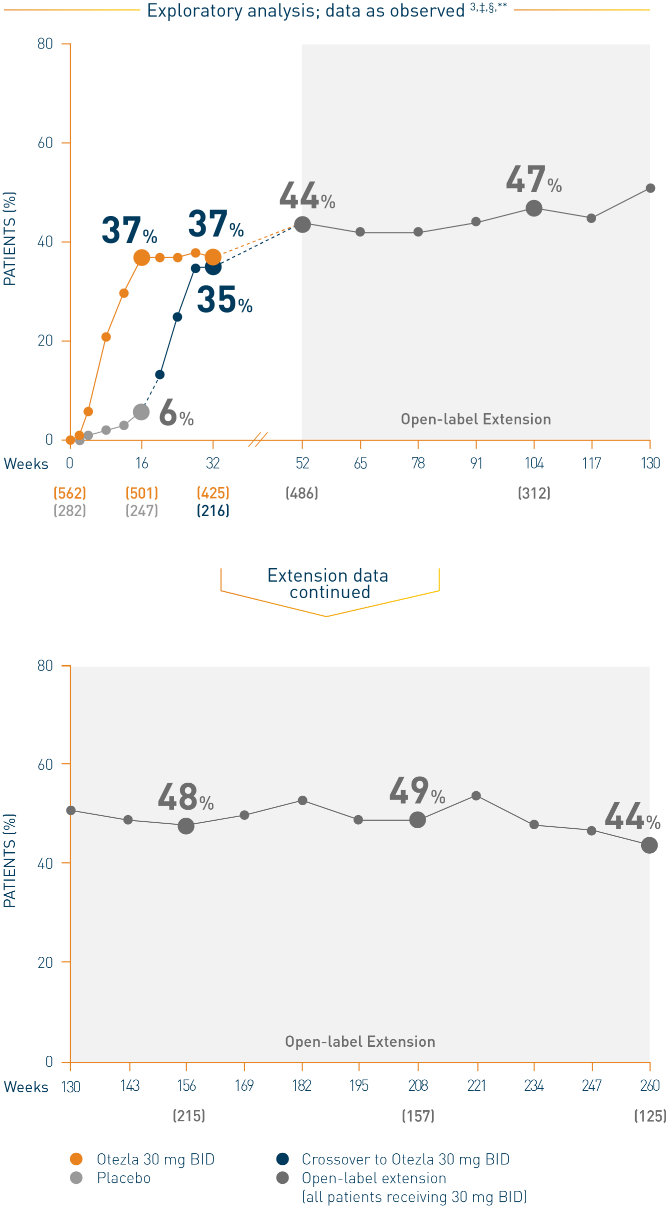
PASI-75 Response with Otezla in the Treatment of Moderate to Severe Plaque Psoriasis — Efficacy | Otezla® (apremilast) Healthcare Professional Site